Abstract
Background: In patients with newly diagnosed (ND) NPM1-mutated acute myeloid leukemia (NPM1 mutAML), presence of measurable residual disease (MRD), as determined by quantitation of NPM1 mut transcripts is an independent prognostic marker (Ivey, NEJM, 2016). MRD assessed by multi-parameter flow cytometry (MFC-MRD) at the end of 2 induction cycles is considered highly prognostic in pts with standard risk NPM1wild type AML (Freeman, JCO, 2018). The value of MFC-MRD is not well-established in patients with ND NPM1 mutAML.
Methods: We examined the prognostic value of MFC-MRD in pts with ND NPM1 mutAML who were treated with intensive chemotherapy (IC; incorporating cytarabine >1000 mg/m 2/d) or low intensity chemotherapy (LIC). MFC-MRD was assessed using an 8-color panel on bone marrow samples obtained at the time of achievement of complete remission (CR), or CR with incomplete count recovery (CRi) [time points for IC : 1-2 months (end of induction), between 3-7 months (consolidation) and ≥ 8 months (completion of therapy); time points for LIC: at the end of 1-2 cycles, and best response]. Sensitivity level was validated at 0.01-0.1%, and negative results were considered valid only if there had been acquisition of at least 200,000 events or a minimum of 200 CD34+ myeloid precursors. Overall survival (OS) was determined from start of treatment until death; relapse-free survival (RFS) from response date to relapse or death due to any cause; pts were censored at the date of hematopoietic stem cell transplant (HSCT) or last follow up. Among 272 pts treated with IC or LIC who achieved CR or CRi, 244 had at least one available MRD assessment and are the subject of this analysis.
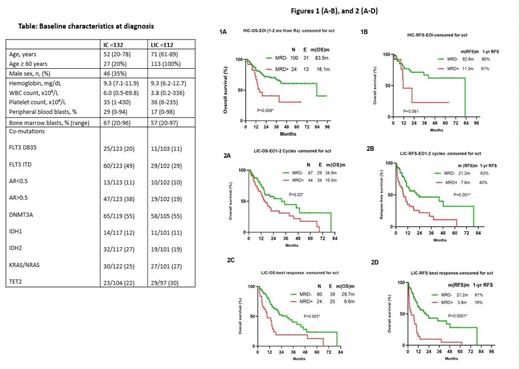
Results: In IC group (n=132), median age was 52 years (range, 20-78 yrs). Median WBC at presentation was 6.0 x 10 9/L (Range, 0.5 - 70 x 10 9/L) (Table). 124 patients had available samples at 1-2 months post induction and 100 (81%) became MRD negative (MRD neg). Achieving MRD neg after induction was associated with a statistically significant improvement in OS (P= 0.006) and a trend towards improved RFS (P=0.06) (Fig 1A & 1B,respectively). Among 101 pts evaluated during consolidation, 89 (86%) became MRD neg. Achieving MRD neg during consolidation was not associated with improvement in RFS (P>0.05) or OS (P>0.05), although the number of MRD positive patients was limited. Thirty patients were evaluated after completion of therapy and 29 (96%) became MRD neg. Among pts with FLT3-ITD co-mutation (n=60), achieving MRD neg at best response was associated with statistically significant improvement in RFS (P=0.0002) and OS (P=0.0002) regardless of the allelic ratio. Among pts who underwent HSCT (n=66), the outcomes were similar between pts who were MRD neg or MRD pos (median OS - NR for both; P>0.05). Among pts who did not undergo HSCT at any timepoint, those who achieved MRD neg had a trend towards better OS than those who remained MRD pos at all time points (83.5 vs 13.3m, P=0.07)
In LIC group (n=112), median age was 71 years (range, 61-89 yrs). Median WBC at presentation was 3.8 x 10 9/L (Range, 0.2 - 336 x 10 9/L) (Table). 112 patients had available samples at the end of 1-2 cycles of therapy and 68 (61%) became MRD neg. Achieving MRD neg at the end of 1-2 cycles was associated with a statistically significant improvement in OS (P= 0.03) and RFS (P=0.001) (Fig 2A & 2B,respectively). Among 104 pts evaluated at best response, 80 (77%) became MRD neg. Achieving MRD neg at best response was associated with a statistically improvement in RFS (P<0.0001) and OS (P=0.003) (Fig 2 C & 2D,respectively).Among pts with FLT3-ITD co-mutation (n=29), achieving MRD neg at best response was not associated with improvement in RFS (P>0.05) and OS (P>0.05) regardless of the allele ratio. Among pts who underwent HSCT (n=19), eleven pts were treated with venetoclax based regimen (n=54) and the outcomes were similar between pts who were MRD neg or MRD pos (82 vs 68.7m; P>0.05). Among pts who did not undergo HSCT at best response, those who achieved MRD neg had significantly better OS than those who remained MRD pos(24.6 vs 9.4m, P=0.003)
Conclusion: Achieving MRD neg-MFC at initial response is associated with a significant improvement in the outcome of patients with NPM1 mutAML who are receiving either intensive or low intensity chemotherapy but in pts with coexisting FLT3-ITD who receive LIC, achieving an MRDneg MFC status may not be associated with significantly improved outcomes but larger studies are needed.
Short: AstraZeneca: Consultancy; Astellas: Research Funding; Novartis: Honoraria; NGMBio: Consultancy; Jazz Pharmaceuticals: Consultancy; Takeda Oncology: Consultancy, Research Funding; Amgen: Consultancy, Honoraria. Kadia: Ascentage: Other; Sanofi-Aventis: Consultancy; Cellonkos: Other; AstraZeneca: Other; Astellas: Other; Genfleet: Other; Pulmotech: Other; Pfizer: Consultancy, Other; Novartis: Consultancy; Liberum: Consultancy; Jazz: Consultancy; Genentech: Consultancy, Other: Grant/research support; Dalichi Sankyo: Consultancy; Cure: Speakers Bureau; BMS: Other: Grant/research support; Amgen: Other: Grant/research support; Aglos: Consultancy; AbbVie: Consultancy, Other: Grant/research support. DiNardo: Forma: Honoraria, Research Funding; Takeda: Honoraria; Bristol Myers Squibb: Honoraria, Research Funding; Novartis: Honoraria; Foghorn: Honoraria, Research Funding; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; ImmuneOnc: Honoraria, Research Funding; AbbVie: Consultancy, Research Funding; Notable Labs: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Agios/Servier: Consultancy, Honoraria, Research Funding; Celgene, a Bristol Myers Squibb company: Honoraria, Research Funding. Konopleva: F. Hoffmann-La Roche: Consultancy, Honoraria, Other: grant support; Cellectis: Other: grant support; Agios: Other: grant support, Research Funding; AstraZeneca: Other: grant support, Research Funding; AbbVie: Consultancy, Honoraria, Other: Grant Support, Research Funding; Ablynx: Other: grant support, Research Funding; Ascentage: Other: grant support, Research Funding; Forty Seven: Other: grant support, Research Funding; Genentech: Consultancy, Honoraria, Other: grant support, Research Funding; Stemline Therapeutics: Research Funding; KisoJi: Research Funding; Novartis: Other: research funding pending, Patents & Royalties: intellectual property rights; Eli Lilly: Patents & Royalties: intellectual property rights, Research Funding; Calithera: Other: grant support, Research Funding; Rafael Pharmaceuticals: Other: grant support, Research Funding; Sanofi: Other: grant support, Research Funding; Reata Pharmaceuticals: Current holder of stock options in a privately-held company, Patents & Royalties: intellectual property rights. Loghavi: Abbvie: Current equity holder in publicly-traded company; Curio Sciences: Honoraria; Gerson Lehrman Group: Consultancy; Guidepoint: Consultancy; Peerview: Honoraria; Qualworld: Consultancy. Issa: Kura Oncology: Consultancy, Research Funding; Syndax Pharmaceuticals: Research Funding; Novartis: Consultancy, Research Funding. Kantarjian: Jazz: Research Funding; Ascentage: Research Funding; AbbVie: Honoraria, Research Funding; BMS: Research Funding; Immunogen: Research Funding; Ipsen Pharmaceuticals: Honoraria; KAHR Medical Ltd: Honoraria; Daiichi-Sankyo: Research Funding; NOVA Research: Honoraria; Amgen: Honoraria, Research Funding; Astra Zeneca: Honoraria; Astellas Health: Honoraria; Aptitude Health: Honoraria; Pfizer: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Precision Biosciences: Honoraria; Taiho Pharmaceutical Canada: Honoraria. Wang: Stemline Therapeutics: Honoraria. Ravandi: Novartis: Honoraria; Agios: Honoraria, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Research Funding; AstraZeneca: Honoraria; Syros Pharmaceuticals: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Xencor: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Astex: Honoraria, Research Funding; Taiho: Honoraria, Research Funding; Prelude: Research Funding; Jazz: Honoraria, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal